Are you about to start gas sensing? Or are you looking for new technology that meets your customers’ specific gas measurement requirements? In this article, you find a complete overview of existing, modern gas detection technologies available on the market, including the latest innovative solutions that are just emerging to overcome common measurement challenges. You discover how each technology works, as well as its primary benefits and potential limitations.
1. Chemiresistive sensors
Chemiresistive sensors use a sensitive conductive material that changes resistance when exposed to gases. These materials can be very diverse like metal oxides, nanoparticles, graphene-based 2D structured materials, polymers,…
1.1. Metal oxide semi-conductor sensors (MOx)
Metal oxide semiconductor sensors are used to detect toxic gases such as carbon monoxide (CO), volatile organic compounds (VOCs), as well as nitrogen dioxide (NO2), and other reducing or oxidizing gases depending on p type or n type. These passive sensors are based on the variation in electrical conductivity of the semiconductor material in the presence of target gases.
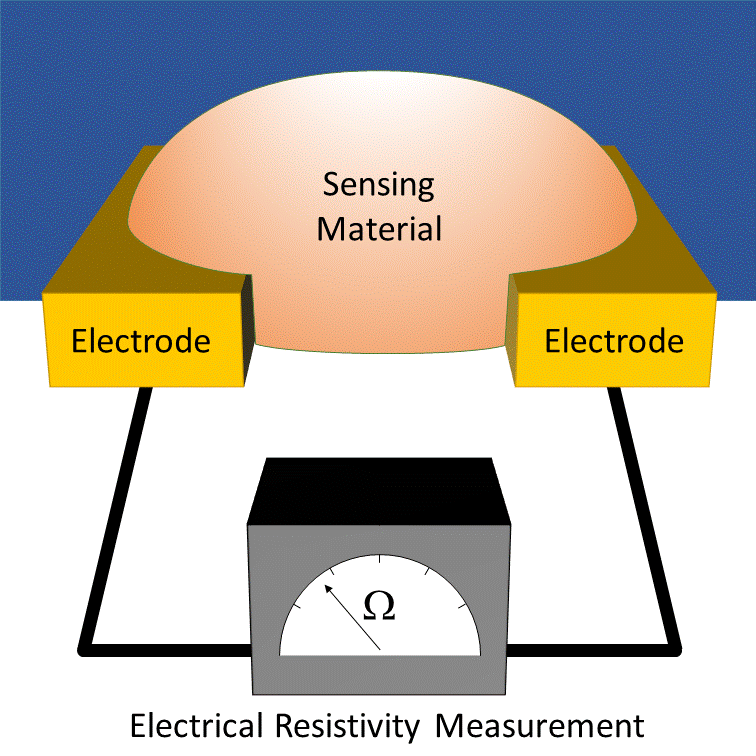
How it works
MOx sensors measure conductivity through a heated transducer often associated to a conditioning circuit. Changes in the ambient environment induce conductivity fluctuations in the semiconducting metal oxide. This requires a high-temperature surface (approximately 200 to 400°C) for gas adsorption and electron exchange. This necessitates a heating circuit (MEMS microheater in microsensor), that significantly increases power consumption.
| Advantages | Disadvantages |
| CMOS-compatible | Low selectivity |
| High integrability thanks to its miniature size | High power consumption |
| Ability to measure low gas concentration | Needs a heater and a conditioning circuit |
| Low price |
1.2. Nanomaterial- and polymer-based sensors
Most nanomaterials and polymers operate at room temperature (no heating circuit is required) and exhibit good resistance to humidity and chemical attack.
How it works
Gases can absorb to the material’s surface, inducing changes in conduction properties, which are detected by resistance variations.
However, like MOx sensors, these sensors may detect multiple gases simultaneously, and the organic or organometallic material can evolve over time. The use of self-calibration algorithms is then recommended to compensate environment and aging-related drifts.
| Advantages | Disadvantages |
| High versatility (choice and application of the sensitive material layer can be adapted depending on the target gas) | Sensitive material may age over time |
| Ultra-low power consumption | Recovery time |
| High integrability thanks to its miniature size | |
| Low price |
2. Eletrochemical sensors (EC)
Electrochemical sensors use electrochemical reactions to detect gases such as carbon monoxide (CO), sulfur dioxide (SO₂) or ammonia (NH₃).
There are two types of electrochemical sensors: amperometric or potentiometric.
How it works
Target gases react with the sensing electrodes, generating an electric current or an electrical potential which is measured and converted into gas concentration. The electrochemical gas sensor reacts with a target gas and produces an electric signal proportional to the gas concentration.
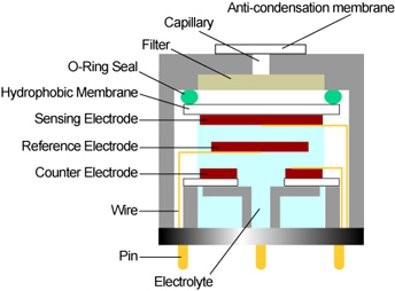
The gas passes through a small capillary opening of the sensor, then diffuses through a hydrophobic barrier and finally reaches the electrode surface. This approach allows enough gas to react with the sensing electrode to produce a sufficient electrical signal while preventing electrolyte from leaking from the sensor.
The gas diffusing through the barrier reacts on the surface of the sensing electrode, involving an oxidation or reduction mechanism. These reactions are catalyzed by electrode materials developed specifically for the target gas. With a resistor connected across the electrodes, a current proportional to the gas concentration flows between the anode and cathode. The current can be measured to determine the gas concentration.
However, the low current value (of the order of nA) requires the use of an analog conditioning circuit (Analog Front End) to convert the current into a voltage (for amperometric EC). This is achieved using a transimpedance amplifier. The output signal of this circuit is directly related to the measured gas concentration. This is measured via an ADC pin of the microcontroller.
| Advantages | Disadvantages |
| Able to measure low gas concentration – at ppb level | Not fully miniaturized (often bulky solution with external analog readout) |
| Selectivity | One sensor per target gas (even if the technology is compatible with a broad gas range) |
| Low power consumption | Limited lifespan (electrolyte consumes itself, like a battery, in contact with target gas) |
| Not easy to handle – asks for environment and sensor chemistry knowledge | |
| High temperature sensitivity | |
| High price |
3. Non-dispersive infrared sensors (NDIR)
Non-dispersive infrared sensors are commonly employed to detect gases such as carbon dioxide (CO₂), sulfur dioxide (SO₂) or methane (CH₄).
How it works
NDIR sensors detect gases by measuring the difference in infrared light at the receptora s the light is absorbed by the gas molecules. Each gas has its own, unique infrared absorption signature, allowing to identify and quantify surrounding target gases.
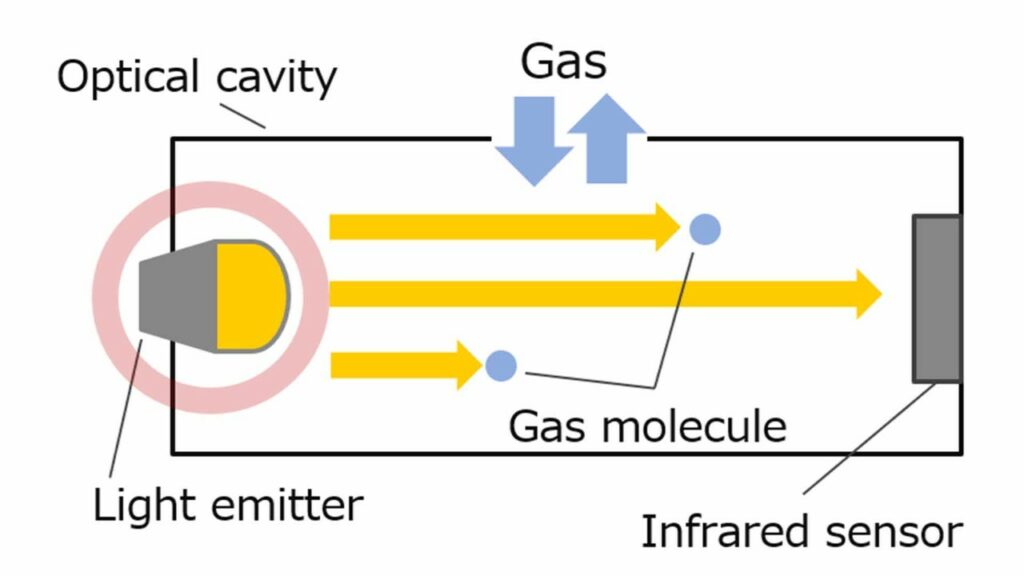
| Advantages | Disadvantages |
| High selectivity | Measure only one gas or one gas family |
| Accuracy | High power consumption |
| Bulky solution |
4. Photoionization detectors (PID)
PID sensors are precise at detecting thousands of VOCs. However, they can not be used to accurately read natural gases (methane, ethane), small molecules (ozone, hydrogen, carbon monoxide, hydrogen…), nor components of clean air (oxygen, carbon dioxide, nitrogen…).
How it works
Photoionization sensors are based on measuring the ionization of gas molecules by a UV light source. When UV light comes into contact with gas molecules, it causes their ionization, that is to say, the formation of positive and negative ions. Ionization is measured by the photoionization sensor which transforms the signal into a gas concentration value.
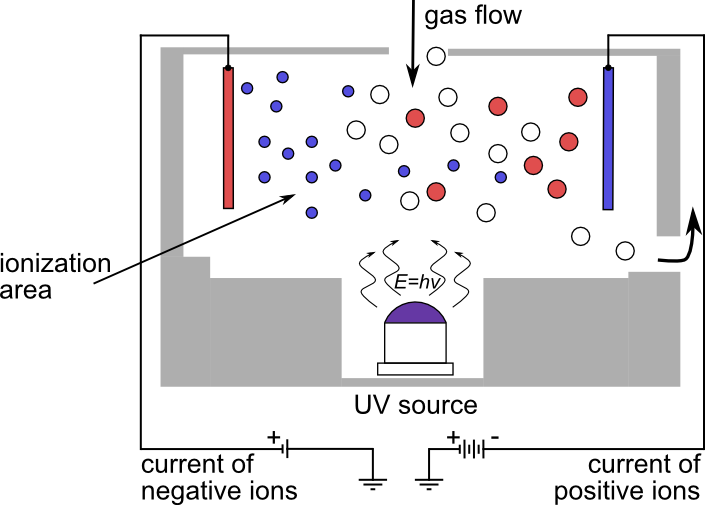
| Advantages | Disadvantages |
| Selectivity to the target gas | High price |
| Ability to detect a large gas range | High power consumption |
| Ability to measure low gas concentration – at ppb level | Sensitivity of the emission source |
| No / low sensor influence | High sensitivity to humidity |
5. Catalytic bead sensors
Catalytic bead sensors measure combustible gases through their thermal conductivity.
How it works
Catalytic bead sensors consist of:
- A detector element, which is sensitive to combustible gases and contains catalytic material
- An inert compensator.
Combustible gases react and burn with the detector element, causing a rise in temperature and resistance.
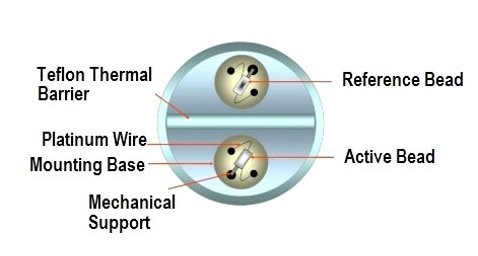
The conditioning circuit often consists of a Wheatstone bridge, limiting the dynamic range (each interface is designed for a specific reacting material).
| Advantages | Disadvantages |
| Low response time | No selectivity |
| Ease of implementation | Limited to explosive gases or with high thermal conductivity variations compare to air or other carrier gas |
| High power consumption (need to be heated) |
Conclusion
Up until the recent development of new, innovative solutions, choosing a gas sensing technology has been highly dependent on specific application requirements, including the gases to be measured, the surrounding environment, and the sensor’s placement within an existing system or infrastructure.
With its EnviCam-3x multi-gas microsensor product line, complemented by AI-based software EnviSoft, VOCSens addresses multiple market needs with a single technology.
We assist you in tackling your gas sensing challenges. Offering versatility, selectivity, precision, and high integrability, coupled with self-calibration capabilities, VOCSens products reduce the need for maintenance operations, making it a valuable choice for your customers’ gas detection needs.




